Cascia
Cascia

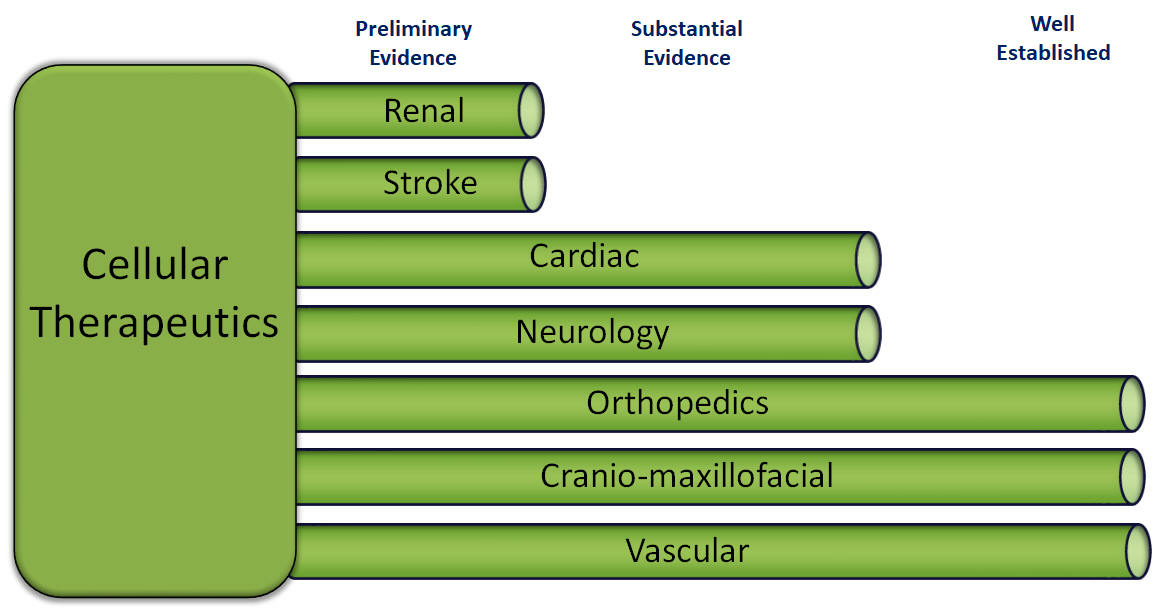
Our cellular therapeutics are prepared from the patient's own cellular material at the hospital where the patient is being treated and thus are regulated as minimally manipulated cell and tissue products, and do not undergo the same approval process as our drugs candidates. Despite the absence of a legal requirement for pre-market approval, it is still necessary for our therapeutics to undergo testing to ensure safety, to optimize our preparation techniques and to accumulate evidence of efficacy for marketing purposes. The acceptance of cellular therapeutics, by both the medical community and insurers, depends on the accumulated evidence rather than a discrete approval event.
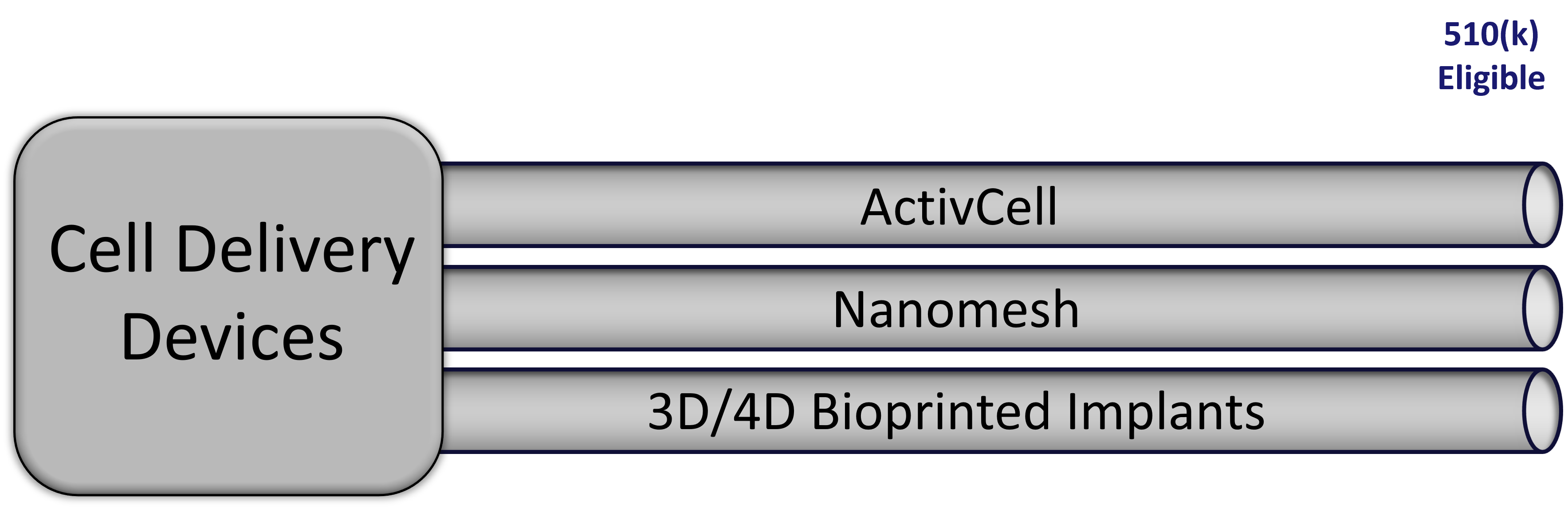
Our devices are custom produced to facilitate delivery of our cellular therapy products, and are regulated as medical devices by the FDA. Medical devices that are substantially similar to products already on the market are eligible for an abbreviated registration process under the Section 510(k) rules, which require a filing with the FDA comparing our product to that of a predicate device already on the market.
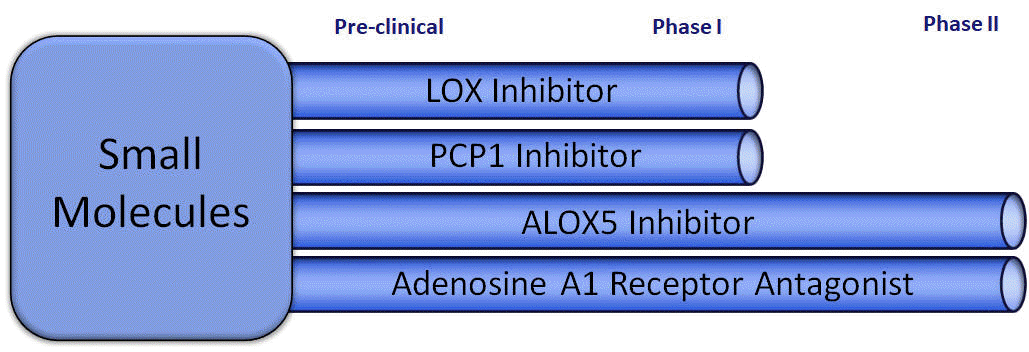
Our small molecule drugs will undergo a more traditional approval process where, after completing a preclinical development process, each product moves through a three-phase testing process prior to approval by the FDA and similar regulatory agencies in other geographies. Phase I testing involves administration of the drug candidate to healthily volunteers, and Phase II testing expands the number of test subjects, and the drug is administered to patients with the condition to be treated. A principal component of Phase II testing is to discover the range of doses where the drug is clearly effective but which are not so large as to cause an undesirable level of side effects or toxicity.
The LOX and PCP1 inhibitors have both undergone extensive preclinical testing and, upon replication of the animal testing, can move directly into Phase I trials.
The ALOX-5 inhibitor has previously been licensed as a drug in a foreign jurisdiction for other indications, and due to its well-established safety profile, can move directly to Phase II testing.
The active ingredient in our Adenosine A1 Antagonist candidate has been used extensively as an adjuvant in literally hundreds of pharmaceutical preparations so it too can begin testing at Phase II.
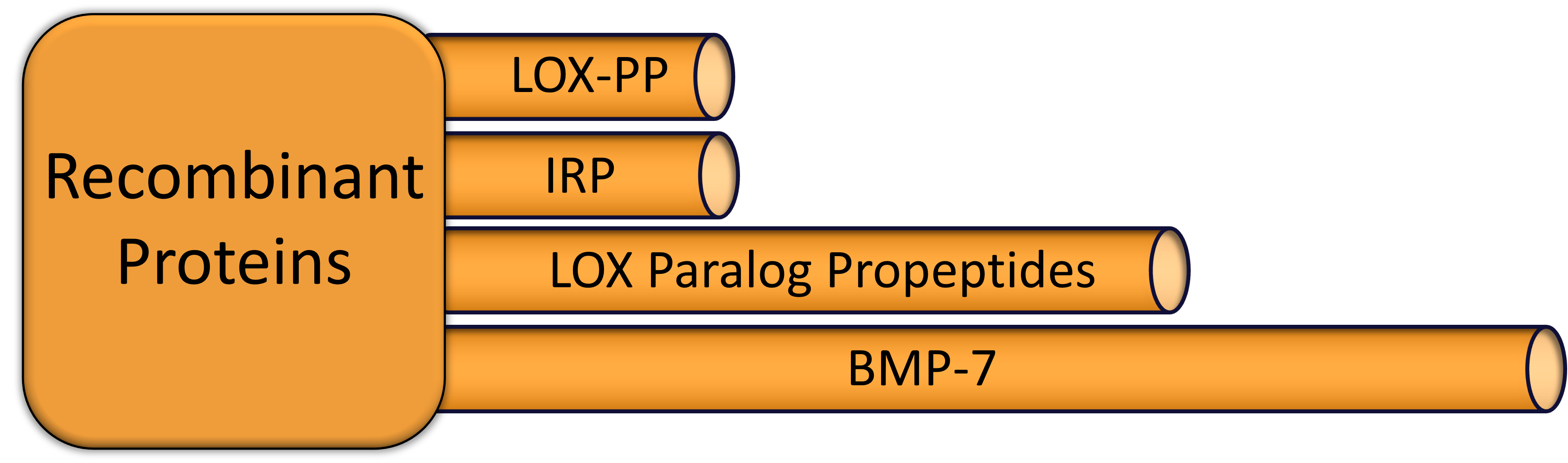
Protein drugs have an approval pathway similar to that of small molecules.
The LOX propeptide has been studied extensively for it role as a potential cancer treatment, but all of that work has been done in animal models. Preclinical work remains to be done to better understand the potential for this drug in treating cancer.
Immunoregenerative peptides (IRP) have been tested in both animal models and a limited number of human subjects.
LOX paralog propeptides have undergone extensive preclinical testing and, upon replication of the animal testing, can move directly into Phase I trials.
BMP-7 is another active ingredient that has been used as a component in other medical products for many years, but the protein itself has never been approved as a monotherapy for any indication. Given the long safety record of the protein, we expect to move directly to Phase II testing.